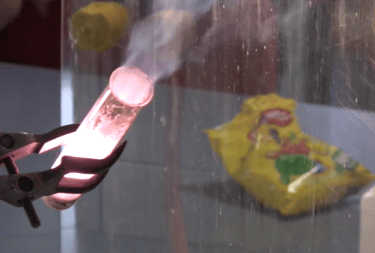Chemistry Geeks Revision
More topics soon!
Chemistry? What's that all about then? I am not sure. That's why this section has so little content. Some say it's "A tiny branch of physics concerned only with the electrons bit of atoms #controversial"? Others claim it is the science version of cooking but with more danger. Others claim the pharmaceutical wonders and materials that will be designed in the coming years will save and transform the entire human race. You decide! (Exit stage left). This is a definite work in progress....bear with us! (Two topics - seriously?). Work shy? Exam tomorrow? Skip to our "Blagger's Guide To Chemistry" section below.
THE BLAGGER'S GUIDE TO CHEMISTRY


There is an awful lot to learn for chemistry and a lot of it involves moles. No, not the furry kind, but mind bending calculations. All those funny symbols and balancing equations too. Still, don't give up! Here is the science geeks tips to get you through those scary chemistry exams.
GCSE Chemistry is another challenging old subject. According to some sources, but not us, we accept no blame, the key five areas for passing GCSE Chemistry are:
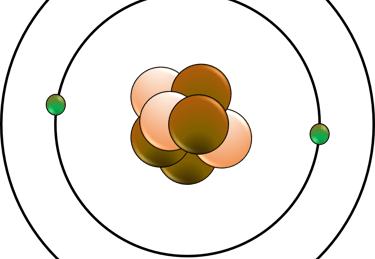
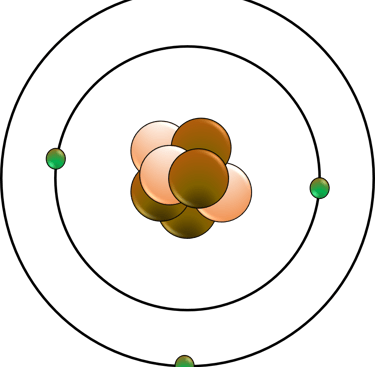
Atomic structure and the periodic table: This area covers the concepts of atoms, isotopes, and ions. You will also learn about the history and development of the periodic table, and how elements are arranged and classified according to their properties and trends.
Bonding, structure, and the properties of matter: Includes the concepts of covalent, ionic, and metallic bonding, and how they affect the structure and properties of substances. You should also learn about the different types of structures, such as simple molecular, giant covalent, giant ionic, and giant metallic, and how they relate to melting and boiling points, electrical conductivity, and solubility. Or not bother, but definitely the firs three,
Quantitative chemistry: This area covers the concepts of relative atomic mass, relative formula mass, empirical formula, molecular formula, moles, molar mass, percentage composition, conservation of mass, balanced equations, limiting reactants, and yield. This is the hard bit. But do not stick your head in the sand....they will turn up!
Chemical changes: This area covers the concepts of acids, bases, alkalis, pH, neutralisation, salts, electrolysis, oxidation, reduction, reactivity series, displacement, and extraction of metals. The difficult bit is learning how to write and balance equations for these reactions. Get the first bit right.
Energy changes: This area covers the concepts of exothermic, endothermic, activation energy, bond energy, and enthalpy change. You will also learn how to measure and calculate energy changes in chemical reactions.
We think you will encounter questions on all of these topic areas. Only a fool would fail to have an overview of each area. Yes, we are rude. But it is for your own good. Bon chance!