IONISING RADIATION
What are the different types of ionising radiation? What is the difference between alpha, beta and gamma radiation? What is positron emission? Which of these types of radiation is the most dangerous to humans? How can those radioactive things hurt us big strong humans anyway? What is meant by half life? What is background radiation? Why are so many things radioactive? Why so many questions? Some of those questions are answered here! Watch our first informative video to find out more and then go to the different sections to learn in greater depth! Oh and we have a great new challenging revision game! Give "Radioactive Crisis" a go. Can you top the leader board?









Which type of radioactive decay can we see?
There are four main types of ionising radiation we need to know about! Alpha, beta minus, positron decay (beta plus) and gamma radiation. All of them are emitted from the nuclei of radioisotopes. But what are they? How ionising are they and what is happening inside the nucleus itself? Hover over the animations to investigate!
Background Radiation

We live on a radioactive planet. The whole earth is a mass of heated radioactive magma and we pathetic humans live on its thin, active crust. From radioactive rocks to radioactive food. From radioactive gas in the ground and air to the fallout from nuclear bomb tests and cosmic rays from space, ionising radiation is everywhere, bombarding our bodies and entering our bones. Watch our most favourite video ever as Professor Ryan Fox roams covid England during lockdown searching for answers.....
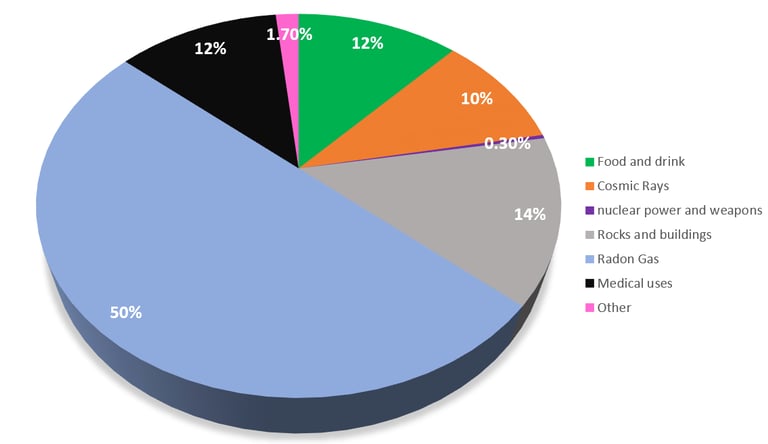
Background radiation is ionizing radiation present in the natural environment. It arises from various sources, both natural and artificial. Let’s explore these sources:
Radon Gas: Radon isotopes contribute to approximately half of background radiation. This gas emanates from the ground and can seep into buildings.
Cosmic Rays: These high-energy particles originate from space. Their exposure is highest at higher altitudes, such as in aircraft or aboard the International Space Station (ISS).
Plants: Some plants absorb isotopes from soil and water, adding to background radiation. They also absorb carbon 14 from the air!
Food: Certain foods, especially those rich in the isotope potassium-40, contribute to background radiation. Plus the plants and animals we eat also contain carbon 14.
Natural Radioisotopes in Water: Radioactive isotopes naturally present in water also play a role.
Rocks and Minerals: Background radiation arises from rocks and minerals, particularly those containing uranium and thorium.
Building Materials: Isotopes in building materials like limestone, concrete, and bricks can emit radiation.
While background radiation is ubiquitous, it usually poses no significant health risk. Human cells have repair mechanisms to handle damage from ionizing radiation.
RADIOACTIVE CRISIS!
Do you now your properties of alpha, beta and gamma radiation? Do you know their dangers? Their uses? Do you know what effect their emission has on the atomic mass and the atomic number? Well. there's only one way to find out! SORT! The sorting game time challenge that is sweeping the nation!
TEST THAT KNOWLEDGE! RADIATION MEGA QUIZ!
Oh look science fans! A challenging quiz all about ionising radiation in it's many forms! Make sure that you know your difference between alpha, beta minus, positron decay and gamma radiation before you get started. Especially the effects of this type of decay on the atomic number and atomic mass. How will you do? Will you get full marks? Go on, have a try...
HALF LIFE
KEY FACTS!
Primary Definition: The time required for half of the radioactive nuclei in a sample.
Alternative Definition: The time it takes for the activity (rate of decay) of a radioactive sample to decrease by half.
Radioactive Decay: The process is random, but the half-life allows us to predict the decay rate.
Measurement Units: Seconds, minutes, hours, days, or years, depending on the radioactive isotope.

Half life gives us some idea of how long a radioactive substance will remain radioactive. But don't be fooled! If something has a half life of 8 minutes, it doesn't mean it will stop being radioactive after that time. It means it will be only half as radioactive as it was when you first encountered it.... confused? You will be! Watch our lovely video to master this difficult concept.
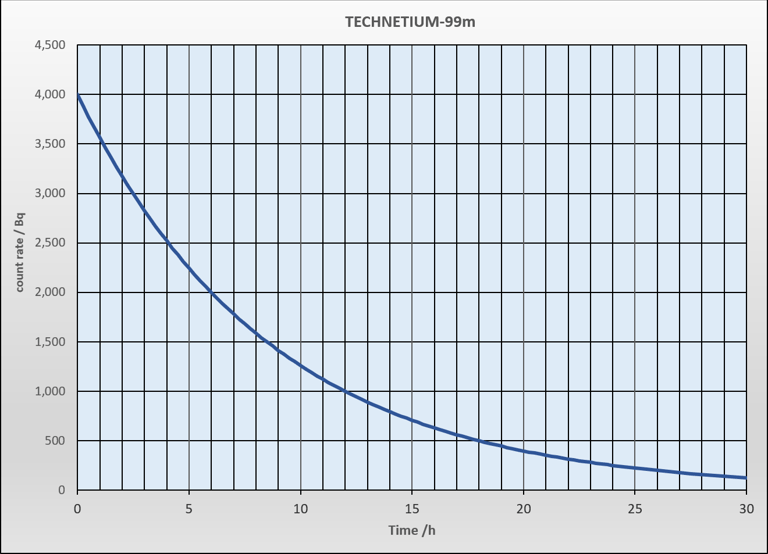
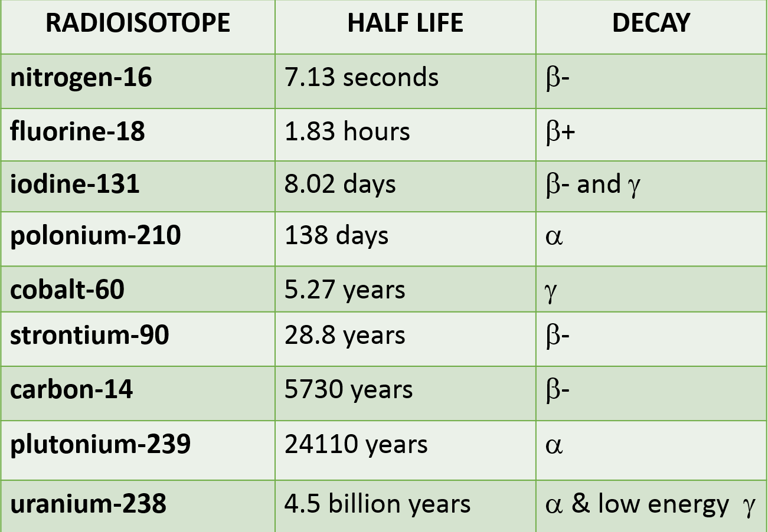
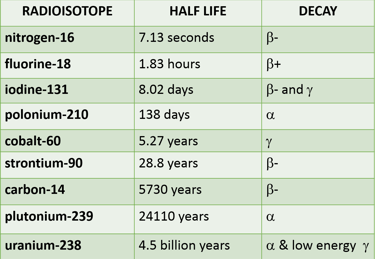
What is the half life of this radioisotope?
Medicine: Used in diagnosis, treatment, and research (e.g., cancer treatment with radiotherapy). It is also used as tracers, for example Iodine-131 has a half-life of about 8 days and is utilized in medical treatments and diagnostics for thyroid conditions.
Archaeology: Dating historical objects and remains using isotopes like Carbon-14 has a half-life of about 5,730 years and is used in radiocarbon dating to determine the age of ancient artifacts.
Geology: Uranium-238 has a half-life of about 4.5 billion years, used in dating rocks and the Earth
Environmental Science: Tracing and studying environmental changes and contamination. Radon-222 for example, is a gassy background radiation seeping up through rocks! It has a half-life of about 3.8 days and is relevant in studying environmental health and indoor air quality.
Nuclear Power: Managing nuclear waste and understanding the behaviour of radioactive materials.
Half-life helps us understand the behaviour of radioactive substances and their practical uses in various fields.
HALF LIFE? WHO CARES?
Who cares? You should dude! I have never said "dude" in my life before. Apologies! It is a vital concept in nuclear safety and medicine! You don't want radioactive substances to be in your body for too long - it will kill you! And as for radioactive waste! It's half life could be tens of thousands of years - what are we going to do with it?










